The authors present their findings on how best to optimise the placement of point-of-care (POC) devices in health networks in resource-limited countries
Introduction
A major constraint in tackling diseases with high socioeconomic burden (e.g., Tuberculosis, Malaria, HIV) in many resource-strapped economies is the unavailability of appropriate diagnostic devices that can inform clinical decisions in a timely manner. Existing devices to diagnose these conditions are usually bulky; require skilled technicians and well-developed infrastructure such as uninterrupted power supply, air conditioning, and sterile environment. These requirements naturally result in formation of centralised diagnostic networks, wherein samples are collected from remote clinics and transported to centralised laboratories and results are communicated back to the clinics. This centralised structure typically leads to inordinate delays between sample collection and return of results to the facilities (termed as turnaround time) and consequently to patients, which in turn leads to poor patient retention and health outcomes.
In response to these challenges, several small and portable devices capable of diagnosing a variety of diseases such as Malaria, Tuberculosis and HIV at the point of care (POC) targeted at resource-limited settings, are under development at various academic research laboratories around the world1. On the one hand, these POC devices obviate the need for complex supply chain for samples and results. However, on the other hand, they are less accurate due to technological limitations. They are also not as inexpensive and easy to operate as “strip tests” such as the ones used to monitor blood sugar levels or detect pregnancy.
Our Model:Incorporating Access vs. Accuracy Tradeoff with Resource Limitations
Very few studies have conducted rigorous comparative evaluation of POC devices with laboratory-based methods. Moreover, these studies employ conventional cost-effectiveness approach to analyse the trade-off between lower device accuracy and lower operational cost2. As a result, they suffer from two main drawbacks with regard to device adoption and implementation within the healthcare network.
First, they fail to incorporate a key benefit of POC devices – improved access or reduced turnaround time to receive test results – which can lead to increased patient retention, improved treatment efficacy, reduced disease transmission and mortality depending on the disease.
Second, the studies do not explicitly account for budgetary constraint, which precludes placement of these POC devices in all facilities in practice.
We overcome these drawbacks by developing an operational model for network-level effectiveness of POC devices, which incorporates the trade-off between improved access and potentially reduced accuracy.
The policy-maker might be better off implementing a lower accuracy device in smaller facilities.
Quantifying the change in access due to POC devices and understanding the effectiveness of deploying such POC devices in resource-constrained health networks, requires careful attention to network externalities implicitly imposed within healthcare networks. Some of the externalities are as follows: Firstly, placing a POC device in one facility could significantly affect the turnaround time at other facilities that do not receive the device due to a modified load at the central laboratory. Secondly, for efficient operation, the central laboratory waits to fulfill the minimum requirement in sample numbers before conducting the tests. Consequently, when faced with a budget constraint (in terms of number of facilities that can receive the POC device), healthcare service providers (or a social planner) must carefully select the location of new POC devices to maximise the overall network level effect on accessibility and thus, health outcomes.
Where in the Health Network to Place the POC Device? That is the Question!
We study the social planner’s problem of maximising the network-level effectiveness by allocating a fixed number of POC devices among candidate health facilities3 and develop an optimisation model to characterise the placement decision. This is important because just having a POC device in a location does not automatically translate to increased effectiveness in healthcare delivery because there could still be inordinate delays for clinics associated with the central laboratory To this end, it is necessary to understand, and model, the operational dynamics of such healthcare service networks and analyse their interaction with the POC device accuracy.
In this research effort, we measure the overall health effectiveness of the network, after placement of POC devices, by the number of quality adjusted life years saved due to treatment, which depends on the number of patients connected to treatment. For clinics with POC devices, we assume that the results are provided with minimal delay and that all patients are connected to care. For clinics that do not get a POC device, i.e., that continue to remain with the centralised laboratory system, we model the number of patients connected to care as a decreasing function of the turnaround time, to capture patient attrition over time.
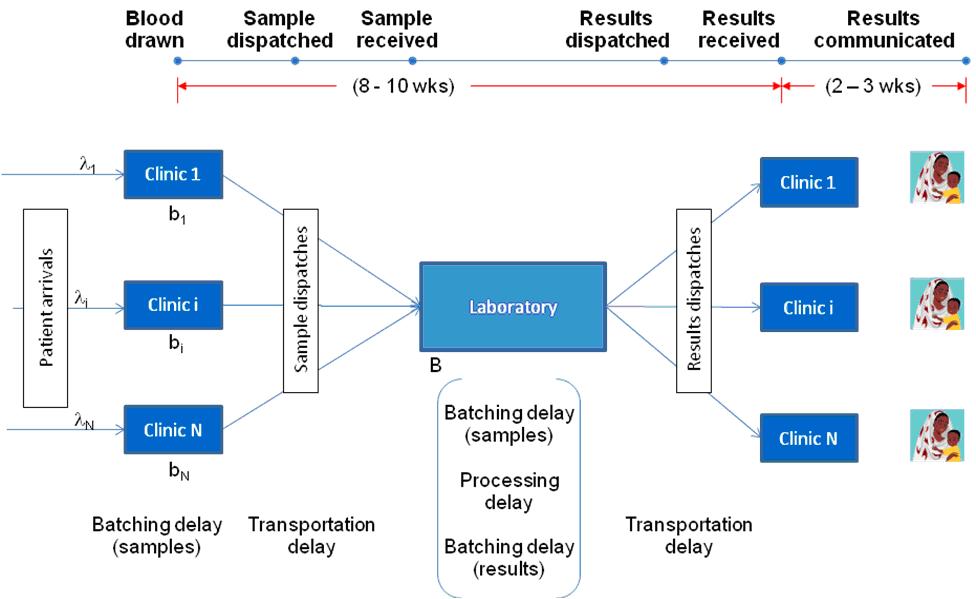
The turnaround time for the centralised laboratory system depends on the operational dynamics of the healthcare network and typically comprises three main components: time to form batches at clinics before dispatching them to the laboratory (i.e., time to collect enough test samples to be shipped to the centralised lab), transportation time from the facility to the laboratory and back, and the time spent in the laboratory for all the samples to be analysed (typically, samples are collected and processed in large batches at centralised facilities to keep costs low). The main sources of uncertainty in this environment are the patient arrivals to the clinic and the availability of transportation opportunities. These uncertainties, in turn, also induce variability in the laboratory operations thereby further adding to the turnaround time.
We capture the resulting operational dynamics of the laboratory using a complex queuing model with both batching and congestion effects. The time spent in the laboratory should increase with reduced sample load due to the batching effect whereas it should decrease due to congestion effect. We then embed this queuing model in an optimisation model, which aims to maximise the network level effectiveness by deciding the appropriate placement of the POC devices. We introduce several modeling innovations and approximations to gain tractability while still capturing the essential elements of the underlying trade-off between increasing access and maintaining accuracy.
We then apply our model to a case study of POC devices for Early Infant Diagnosis (EID) of HIV. Figure 1 illustrates such a centralised laboratory network for diagnosing HIV infection in infants. We populate our model using operational data on an EID network from an East African country, technical data on a POC device being developed at a US university, and clinical data from secondary sources in the medical literature. We use our modeling framework to evaluate simple rules of thumb for device placement, typically used by healthcare practitioners such as allocating devices to facilities with largest disease prevalence or to those with largest patient load. We find that the relative effectiveness of these thumb rules compared to the optimal solution depends on both the device characteristics (accuracy) and the network characteristics (level of congestion). Specifically, we characterise conditions under which these relatively simple thumb rules are, in fact, optimal. For example, allocating POC devices to facilities with the highest prevalence rate is optimal if the facilities have similar patient volume and the sensitivity of the POC device is sufficiently high. Similarly, allocating POC devices to facilities with the highest volume rule is optimal if the facilities have similar prevalence rates and the service rate of the laboratory is sufficiently small. Moreover, our analysis also provides relative ranking of these thumb rules for different device and network characteristics. For instance, placing the POC devices in facilities with the largest patient load outperforms placing them at facilities with the largest prevalence if the accuracy of the device is sufficiently high and vice versa. This implies that the policy-maker might be better off implementing a lower accuracy device in smaller facilities. Finally, our model and analysis could also be used to quantify the magnitude of potential welfare loss resulting from implementation of these practice-based thumb rules.
Overall, in this research effort we highlight the importance of jointly optimising over-allocation of POC devices and reassignment of remaining health care facilities to the centralised laboratories, i.e., accounting for the network externality imposed when POC allocation decisions are made. While, in our experience, these decisions are not necessarily taken jointly by healthcare practitioners, integrating operational dynamics in such decision-making can vastly improve health outcomes, especially in resource-constrained environments.